Healthcare logistics and temperature-sensitive healthcare products
15 December 2021 by Edina GÁLFI
15 December 2021 by Edina GÁLFI

Temperature-sensitive healthcare products
Under Article L5311-1 of the French Public Health Code, human health products are divided between these categories:
Each category of products is then subject to strict storage regulations governed by two main parameters: temperature and duration. The authorities in charge of granting marketing authorisations and the French national agency for the safety of medicines, the ANSM, will then determine the regulatory temperature ranges based on the active substances, molecules and technologies used.
Focus – Health products in a few figures :
Today in France, there are 500 such products in the “cold” category, representing 1 to 2% of the volumes of medicinal products. Vaccines, for their part, represent only 11% of medicinal product volumes. Finally, blood products are supplied by the 3 million blood donations made each year to France’s 17 collection centres.
The feats of research, the race to biotechnological innovation and the globalisation of trade have placed the logistics challenge at the heart of large-scale pharmaceutical projects. The COVID 19 pandemic has provided us with a perfect example of this. It is therefore instructive to consider the impact of effective cold chain management on the logistics of temperature-sensitive healthcare products.
Shelf-life and temperature during the storage and transport of healthcare products are closely linked. In fact, the shelf life and efficacy of an active substance will be considerably reduced if the regulatory conservation temperature is not respected.
The right conservation temperature is established after conducting a stability study. Nevertheless this step is long and costly and, as a result, often cut short. And so the conditions of conservation are often over-optimised to avoid any health risk. Certain products therefore find themselves being conserved at -80°C when conservation at +4°C would have been recommended if the study had been done. Such inappropriate solutions can therefore quickly become costly.
The regulations allow for a temperature excursion during the transfer of active substances from one container to another of 3 minutes maximum. After that, there is a risk to the health safety of the products. It therefore necessary to distinguish between two types of risk factors: those due to heat and those due to cold.
Healthcare logistics is above all governed by a performance obligation, i.e. that healthcare products stored and transported must be viable upon arrival. That their efficacy and potency must not have been altered by logistics-related factors. There are no pharmaceutical regulations other than the Guidelines on Good Distribution Practice that impose any requirements regarding the means used to store and transport healthcare products. Nevertheless, multiple solutions exist in cold logistics.
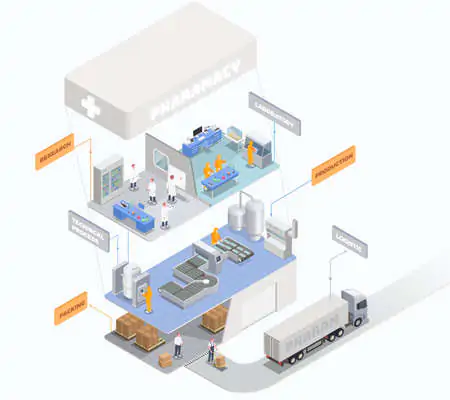
The use of the insulated container rests on the principle of pooled logistics. In a process of logistics optimisation, several different temperature ranges can be transported on the same, standard lorry. In this way, each container transported can be dedicated to the transport of a different category of temperature-sensitive healthcare products. It is therefore possible to transport products at -80°C, -20°C, +2°C/+8°C and even +15°C/+25°C on the same lorry.
This solution for cold logistics can be part of a sustainable approach since it also allows you to employ reverse logistics, which is both more environmentally friendly and more economical. The quantity of passive cooling is then adapted to the length of time the temperature needs to be maintained. Thus, a customised, economical approach is available to the different actors in the supply chain.
And it is a combination of a rotomoulded insulated container technology 100% Made In France and a passive cooling solution that guarantees the successful transfer of temperature-sensitive healthcare products.
